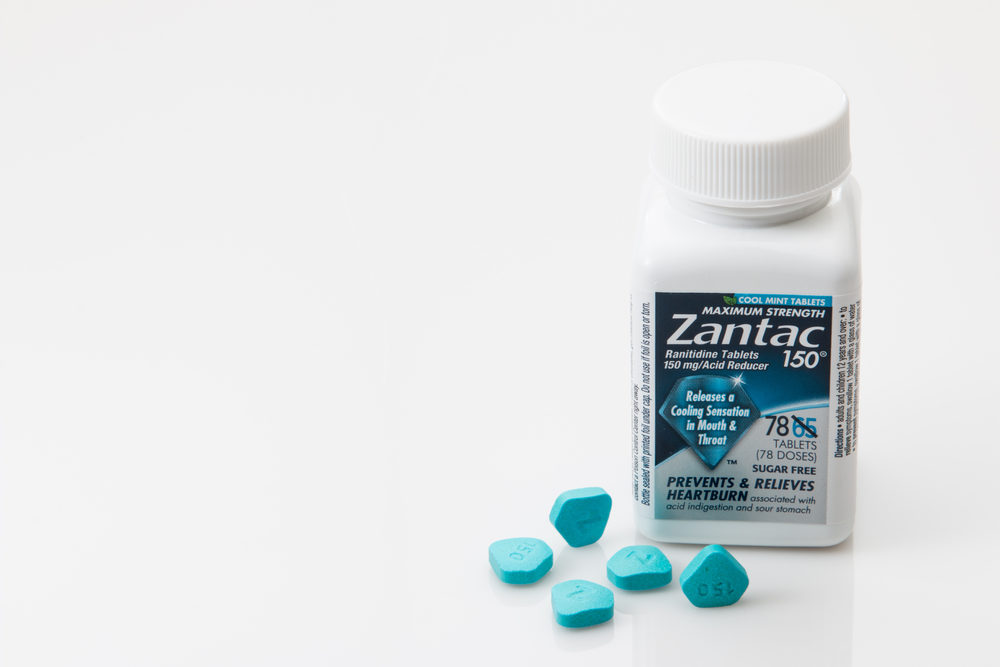
FDA approval for Zantac as a short-term treatment of a common form of. It is an H2 histamine antagonist.
 Weekly Dose Ranitidine The Heartburn Medicine Being Recalled Because Of Cancer Causing Contamination
Weekly Dose Ranitidine The Heartburn Medicine Being Recalled Because Of Cancer Causing Contamination
It joins the 1 billion antacid market as the third of.
When did zantac come out. Studies pointing to risk date to 1980s. French drug giant Sanofi won approval beginning in 1995 to sell the branded Zantac without a prescription. At this point over 31 countries had approved Zantac for use.
Glaxos Zantac began its dominance of the acidpeptic marketplace with a launch strategy taking advantage of the established Roche sales force to rapidly promote the product. The following is a timeline on Zantac. Zantac 75 tablets half the strength of prescription Zantac will be on pharmacy shelves early next year manufacturer Glaxo Wellcome said.
The FDA asked manufacturers to. The Food and Drug Administration has recalled the popular heartburn medication ranitidine known by the brand name Zantac. Zantac was a very popular antacid drug since the 1980s.
Originally sold in Britain and Italy in 1981 Zantac first obtained US. On April 1 2020 the FDA requested manufacturers to withdraw all prescription and over-the-counter OTC ranitidine drugs Zantac others from the market immediately due to the presence of a contaminant known as N-Nitrosodimethylamine NDMA. FDA approval in 1983.
In 2016 Stanford University researchers tested urine samples of 10 people who took a 150-milligram tablet of Zantac and found NDMA levels far greater than. Glaxo Holdings now GlaxoSmithKline launched Zantac as a competitor to the highly-successful ulcer drug Tagamet which was introduced in 1975. According to Davison Zantac is an H2 blocker.
Although the FDA did not observe unacceptable levels of NDMA in many of the samples they tested they have determined that. Sanofi has halted distribution in Canada where health officials required such a move but. Zantac is not a proton pump inhibitor.
It is extremely effective for some people and has been considered a safe and trusted medication for decades. It is an old medication and has been around for years. Its generic name is ranitidine.
FDA approval for Zantac as a short-term treatment of a common form of. What is Zantac. Educational symposia for physicians were instrumental in disseminating both disease and product information to primary care physicians and specialists.
Zantac 300 mg tablets from GlaxoSmithKline GSK Ranitidine was first prepared in England as AH19065 by John Bradshaw in the summer of 1977 in the Ware research laboratories of Allen and Hanburys part of the larger Glaxo organisation. It is also not useless as stated in another answer. Glaxos histamine H2 receptor antagonist Zantac ranitidine has received its first approval in the.
It was on the market for 36 years before it was taken off the market in 2019. 1983 Glaxo Holdings Ltd now a part of GlaxoSmithKline PLC receives its first US. Moreover patient response rates.
Glaxo Holdings Ltd a company that is now part of GlaxoSmithKline PLC received approval from the FDA to sell Zantac. Glaxo Holdings Ltd which is now known as GlaxoSmithKline first sold Zantac in 1983. 1983 Glaxo Holdings Ltd now a part of GlaxoSmithKline PLC receives its first US.
The US trial indicated that Zantac 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The following is a timeline on Zantac. It was the 50th most prescribed drug with 15 million prescriptions a year.
The improvement was maintained throughout the 6-week trial period. There are several manufacturers of the generic form of this medication called ranitidine Why has it been recalled. Ranitidine-treated patients consumed significantly less antacid than did placebo-treated patients.
It blocks the signal to the stomach to make acid. To continue reading The Pharma Letter please login subscribe or claim a 7 day free trial subscription and access exclusive features interviews round-ups and commentary from the sharpest minds in the pharmaceutical and. Millions more who purchased the drug and its generic equivalent over-the-counter.
The first studies about the NDMA in ranitidine came out in the early 1980s and its taken until now for the drug to be pulled. Wrong information was given in another answer. In 1988 Zantac became the worlds best-selling drug and one of the first drugs to reach 1 billion in sales.
This technique not only pleased physicians more referrals but also. According to Wired as of 2020 15 million Americans were taking ranitidine at prescription levels with millions more taking lower doses of the drug. The drugs first approved usage was for the short-term treatment of ulcers.
Prescription-strength Zantac is used for the treatment of very severe heartburn and more serious conditions such as stomachintestinal ulcers. Zantac is a medication that reduces the production of stomach acid. This drug was originally developed by.









/what-causes-a-burning-throat-17429901-5b44da70c9e77c00376783f3.png)



/can-i-collect-unemployment-if-i-am-fired-2064150-v2-5babf19246e0fb0025a74968.png)